
What is ALL?
ALL is a cancer of the blood and bone marrow that affects the white blood cells in the bone marrow and blood.
Here’s What Happens in ALL
Too many immature white blood cells (also called lymphoblasts or leukemic cells) are made in the bone marrow.
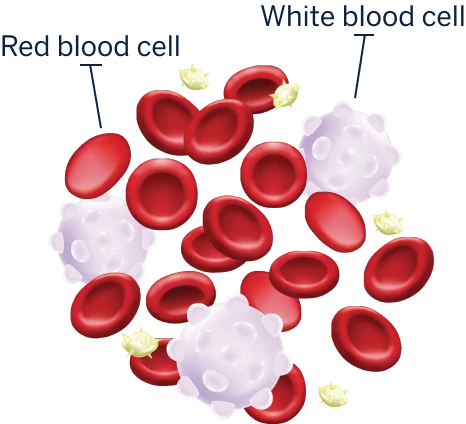
NORMAL BLOOD
LEUKEMIA
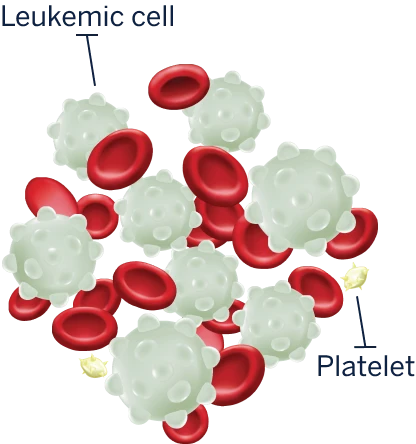
These white blood cells don’t work properly to fight infections. They grow more quickly and live longer than normal cells, so they crowd out the healthy blood cells the body needs.

Eventually, all of those immature cells spill out into the bloodstream and begin to cause problems elsewhere in the body, such as in the spleen, thymus, lymph nodes, liver, testicles, and the central nervous system.
Who is affected by ALL?
Although ALL is the most common type of childhood leukemia, it can also affect people between the ages of 15 and 39 years—often called adolescents and young adults (AYAs)—as well as older adults.
The median age at diagnosis is
17 years
46% of new ALL cases occur in people aged
≥20 years
If you have ALL and are within the AYA age group, you know that this is a time in your life for making plans and looking forward to your future.
Here are a few things to think about when discussing treatment options with your healthcare team:
Even if you’re not thinking about having children now, it could be something you want in the future. Talk to your doctor about how different drugs included in your chemotherapy regimen may affect having children, and how you can preserve your fertility.
Think about how treatment will integrate into your daily life, such as getting to appointments with your healthcare professional and being able to keep up with work or school.
When talking with your healthcare team about treatment, ask about the side effects that may affect you even long after treatment has ended.
Read about how ONCASPAR is a key part of treatment for ALL
