
For more than 50 years, the active ingredient in ONCASPAR, asparaginase, has been shown to be an important part of how acute lymphoblastic leukemia (ALL) is treated.
Here’s how ONCASPAR works
Cancer cells need fuel to keep growing and dividing. In ALL, the cancer cells are fueled by asparagine, a substance that is found in the body. ONCASPAR is thought to break down asparagine and starve the cancer cells so they can no longer grow and divide.
ASPARAGINE
Cells need an amino acid called asparagine to survive.
LEUKEMIC CELL
Healthy cells make their own asparagine, but ALL cells have to get asparagine that’s circulating in the blood.
ONCASPAR, which is a type of asparaginase, is thought to break down the asparagine that’s circulating in the blood, so the leukemic cell can’t get what it needs.

Asparagine
Asparaginase
Broken down
asparagine
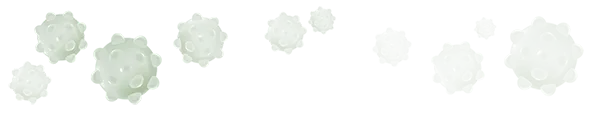
Dying leukemic cells
Without a source for this vital substance, the leukemic cells die.
Keep reading about how ONCASPAR can help
